Where are we in finding a treatment for COVID-19?
Some drugs and treatments have shown promise in clinical trials, while others are drawing controversy.

Editor’s Note: This series is produced in partnership with the World Health Organization (WHO).
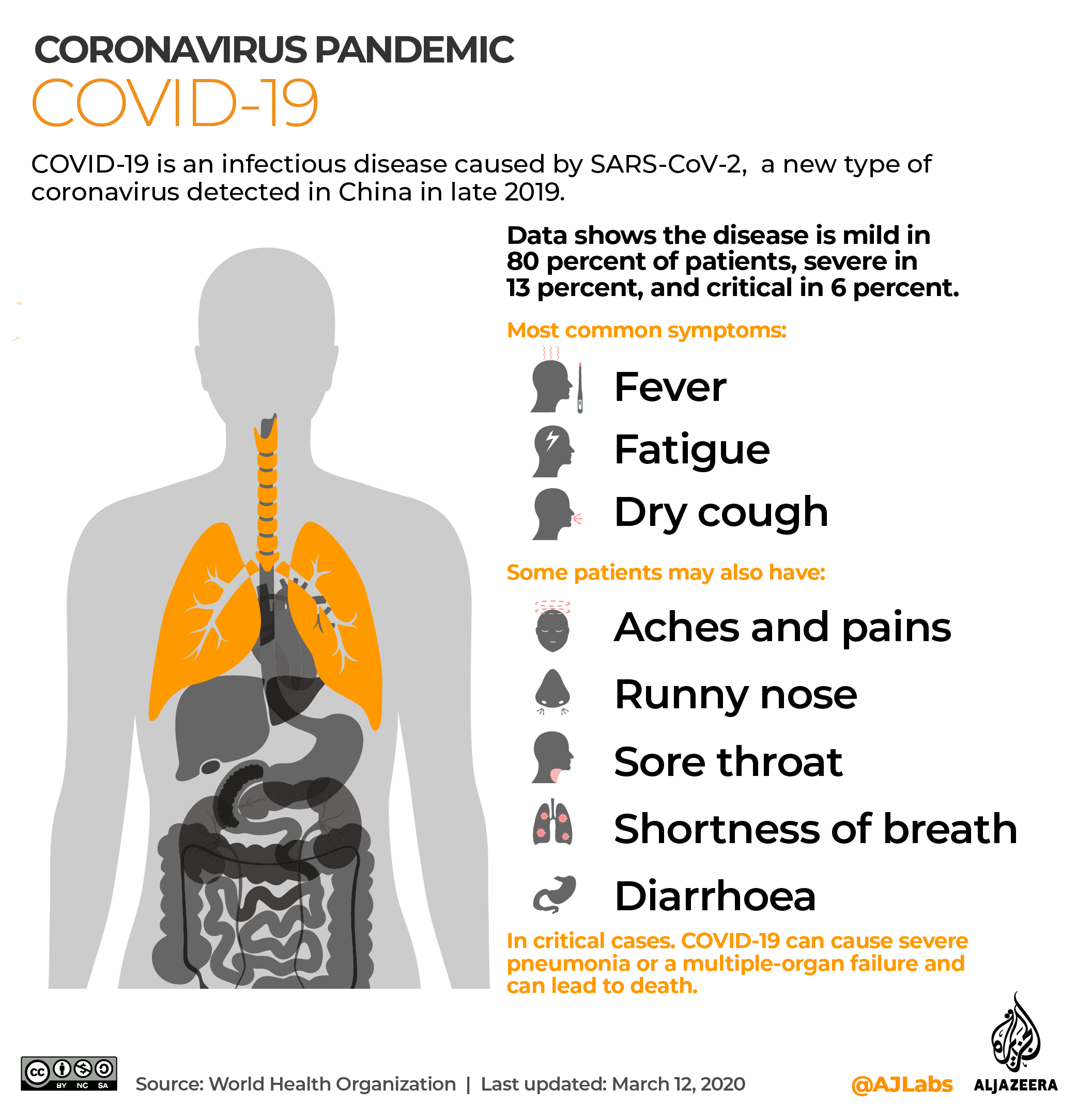
The novel coronavirus has infected more than 18 million people worldwide – of which 10 million have so far recovered, while at least 690,000 have died.
Keep reading
list of 3 itemsQandA: Airborne COVID-19 spread possible, risky in some settings
Face masks and COVID-19: All you need to know
There are currently no licensed treatments nor a vaccine for COVID-19 – the disease caused by the new coronavirus – but several drugs are being studied in large clinical trials and more research is under way.
In the United States, a study funded by the National Institutes of Health (NIH), has reported that the antiviral drug remdesivir, which is administered intravenously in hospital, provided some improvement in clinical recovery among patients requiring supplemental oxygen. More information on the efficacy of remdesivir in patients with COVID-19 is expected from large clinical trials such as the WHO-coordinated Solidarity Trial and the Recovery Trial in the United Kingdom.
Meanwhile, according to the results from a UK study, a cheap and widely-used steroid called dexamethasone, given either orally or intravenously, has been shown to reduce mortality by about one-third in coronavirus patients on ventilators and one-fifth in those who require oxygen.
The WHO is expected to publish updated treatment guidelines on dexamethasone and other corticosteroids.
“It’s really important that these drugs be evaluated in robust clinical trials before being used because they can put the patient’s health at risk,” Silvia Bertagnolio, medical officer at the WHO, told Al Jazeera.
“We are very closely monitoring the evolution of clinical trials and we make recommendations as we go,” she added.
How to treat COVID-19 patients
In the absence of approved therapeutics, many countries are using experimental or repurposed drugs to treat coronavirus patients, which is called the “off-label use” of medicine, Bertagnolio said.
However, the WHO strongly warns that off-label drugs should only be used in the context of controlled studies, preferably randomised controlled trials to assess efficacy and safety.
According to Soumya Swaminathan, WHO’s chief scientist, about 10 to 15 percent of COVID-19 patients develop severe illness with breathlessness, signs of sepsis and cardiac problems, and they need to be admitted to hospital.
The vast majority have mild to moderate symptoms, or do not show any symptoms, meaning they are asymptomatic.
Patients with mild cases, while isolating at home, may take paracetamol to reduce the fever, Bertagnolio said.
Antibiotics, which work only against bacteria, not viruses, are not recommended to treat or prevent COVID-19.
However, if you are hospitalised for COVID-19, antibiotics may be helpful in case of bacterial co-infection or pneumonia.
Antithrombotic drugs to treat or prevent blood clots, and monoclonal antibodies, which prevent the virus from binding to the cell and entering the cell, are also being assessed.
“There are different phases of this disease and the treatment really needs to be tailored to each stage,” Swaminathan said during a live Q&A on YouTube.
“In the earlier stages, the antivirals might have a better effect and in the later stages, once there is already lung damage, you would need to damp down the inflammatory response, use antithrombotics and good supportive care,” she added.
|
|
Controversial remedies
The use of anti-malarial drug hydroxychloroquine, touted by US President Donald Trump and his Brazilian counterpart Jair Bolsonaro, has drawn controversy and divided opinions.
Clinical trials have shown that hydroxychloroquine does not reduce deaths nor provide clinical benefits among hospitalised COVID-19 patients, and there is currently no data about the drug’s ability to prevent COVID-19.
The United States Food and Drug Administration (FDA) has warned against taking hydroxychloroquine or chloroquine outside of a hospital or formal study citing serious heart rhythm problems and other safety issues, including blood and lymph system disorders, kidney injuries, and liver problems and failure.
The WHO says the misuse of hydroxychloroquine, generally safe for patients with malaria and autoimmune diseases, can cause serious side effects, including heart disease or arrhythmia.
Alternative remedies are also being used in some countries, such as the herbal tonic named Covid-Organics, launched by the Madagascan president, and a bleach-like agent – chlorine dioxide – approved by the Senate in Bolivia.
Health authorities have cautioned against the use of such untested remedies.
“There are no in vitro studies even indicating the potentiality of these drugs, therefore we have to be very cautious because they can be more of a harm to the patient and provide no benefit,” Bertagnolio said.
Follow Saba Aziz on Twitter: @saba_aziz