J&J submits one-shot COVID vaccine for WHO emergency use approval
Johnson & Johnson says an emergency use listing is critical for supplying vaccines to poor and middle-income countries through the COVAX programme.
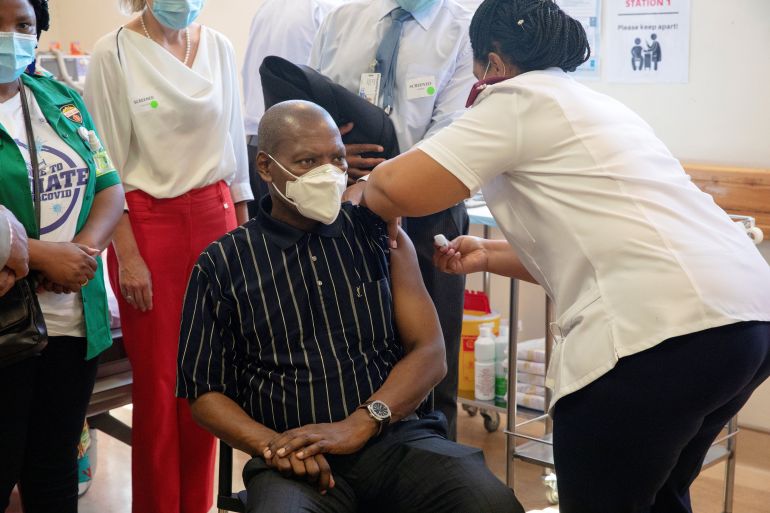
Johnson & Johnson said on Friday that it had submitted data to the World Health Organization (WHO) for emergency use listing of its COVID-19 vaccine, which would allow for wider access to the one-dose shot.
J&J said an emergency use listing is a prerequisite for supplying vaccines to the COVID-19 Vaccines Global Access Facility (COVAX) programme, co-led by WHO, which aims to deliver doses to poor and middle-income countries.
Keep reading
list of 4 itemsIsraeli study finds Pfizer vaccine 85% effective after first shot
South Africa launches vaccine roll-out with Johnson & Johnson jab
Johnson & Johnson asks US to approve single-dose COVID jab
The J&J vaccine is administered in a single dose and can be stored at normal refrigerator temperatures, a big selling point in countries with relatively weaker healthcare infrastructure.
The vaccine is under review by the United States health regulator, and a panel of the US Food and Drug Administration’s experts are expected to discuss the vaccine’s emergency use authorisation next week.
The vaccine is being rolled out in South Africa, for the first time outside a major clinical trial.
J&J said last month that the vaccine was 66 percent effective in preventing COVID-19 in a large late-stage global trial against multiple variants of the coronavirus. The level of protection of the vaccine varied from 72 percent in the US, to 66 percent in Latin America and 57 percent in South Africa.
The company said the data delivered to WHO includes results from the late-stage trial.
J&J entered into an agreement in December in support of the COVAX programme.
The company and Gavi, the Vaccine Alliance – which also co-leads COVAX – expect to enter into an advance purchase agreement that would provide up to 500 million doses of the single-dose vaccine to COVAX through 2022, J&J said.