US FDA authorises Pfizer COVID-19 jab for kids aged 5 to 11
Food and Drug Administration grants emergency authorisation for the Pfizer-BioNTech coronavirus vaccine in children.
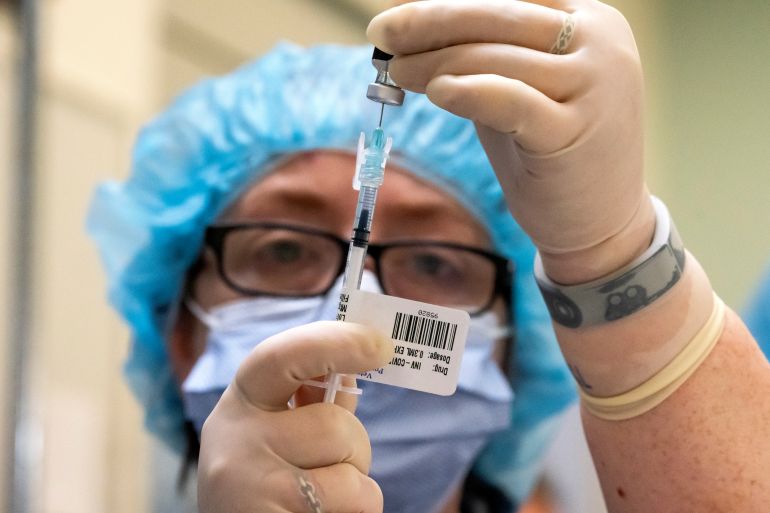
The US Food and Drug Administration (FDA) has granted emergency authorisation for the Pfizer-BioNTech coronavirus vaccine to be given to children between the ages of five and 11 years.
It is the first coronavirus vaccine approved in the United States for young kids, after an overwhelming vote from a panel of FDA advisers earlier this week.
Keep reading
list of 4 itemsMexico’s teachers seek relief from pandemic-era spike in school robberies
‘A bad chapter’: Tracing the origins of Ecuador’s rise in gang violence
Why is the US economy so resilient?
Friday’s approval is expected to make the vaccine available to 28 million American children, many of whom are back in school for in-person learning.
Pfizer and BioNTech said their vaccine showed 90.7 percent efficacy against the coronavirus in a clinical trial of children aged five to 11.
“Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Dr Janet Woodcock, the acting FDA commissioner, said in a statement. “Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards.”
Only a few other countries, including China, Cuba and the United Arab Emirates, have so far cleared COVID-19 vaccines for children in this age group and younger.
An advisory panel to the US Centers for Disease Control and Prevention (CDC) is scheduled to meet on Tuesday to consider how the Pfizer-BioNTech vaccine should be used in that age group. The CDC director will have the final say on approving it.
With the FDA’s decision, Pfizer plans to begin shipping millions of vials of the pediatric vaccine — in orange caps to avoid mix-ups with the purple-capped doses for everyone else — to doctors’ offices, pharmacies and other vaccination sites. Once the CDC issues its ruling, eligible kids will get two shots, three weeks apart.
While children are at lower risk of severe illness or death from COVID-19 than older people, five- to 11-year-olds still have been seriously affected, including more than 8,300 hospitalisations, about a third requiring intensive care, and nearly 100 deaths since the start of the coronavirus pandemic, according to the FDA.
Nearly 70 percent of five- to 11-year-olds hospitalised for COVID-19 in the US have other serious medical conditions, including asthma and obesity, according to federal tracking. Additionally, more than two-thirds of youngsters hospitalised are Black or Hispanic, mirroring longstanding disparities in the disease’s effect.
Pfizer and BioNTech expect data from a clinical trial in children ages two to four years by yearend.
The US started vaccinating teens between ages 12 and 17 in May. Vaccination coverage among children in that age group is lower than in older groups, according to the CDC.
Pfizer’s vaccine was the first to be authorised for emergency use in the US in December 2020 for those aged 16 and older. It gained clearance for the 12 to 15 age group in May and was granted full US approval in August.
Some US parents are expected to vaccinate their young children before family holiday gatherings and the winter season.
But a recent Kaiser Family Foundation survey suggests most parents will not rush to get the shots. About 25 percent of parents polled earlier this month said they would get their children vaccinated “right away”.
But the remaining majority of parents were roughly split between those who said they will wait to see how the vaccine performs and those who said they will “definitely” not have their children vaccinated for COVID-19.